In collaboration with PM Buston, J Atema (Boston University), CB Paris (U. Miami). Funded by NSF – Ocean Sciences Collaborative Grant: #1459224 to JFW, #1459546 to PM Buston, and #145156 to C Paris.
Understanding the patterns and causes of larval dispersal and their consequences for fish populations is a major goal of 21st century marine ecology. Patterns of larval dispersal determine the probability of larval exchange, or connectivity, among populations. In turn, population connectivity has major consequences for metapopulation dynamics and evolution within metapopulations. Further, understanding population connectivity is critical for the design of effective networks of marine reserves and for development of sustainable fisheries. This project entails a comprehensive, integrated, and potentially transformative investigation of how marine larvae orient in the pelagic environment in order to find suitable settlement sites. It is being done using the neon goby, Elacatinus lori and there are three motives for the choice of study system: i) direct genetic methods have already been used to describe the complete dispersal kernel for this species, and these observations indicate that dispersal is less extensive than predicted by a high-resolution biophysical model; ii) E. lori can be reared in the lab from hatching to settlement providing a reliable source of larvae of all ages for proposed experiments; iii) a new, proven behavioral observation platform, the Drifting In Situ Chamber (DISC, pioneered by Dr. Paris), allows measurements of larval orientation behavior in open water.
This collaborative project had three objectives: 1) to understand ontogenetic changes in larval orientation capabilities by correlating larval orientation behavior with developmental sensory anatomy; 2) to analyze variation in the precision of larval orientation in different environmental contexts through ontogeny; 3) to test alternative hypotheses for the goal of larval orientation behavior.
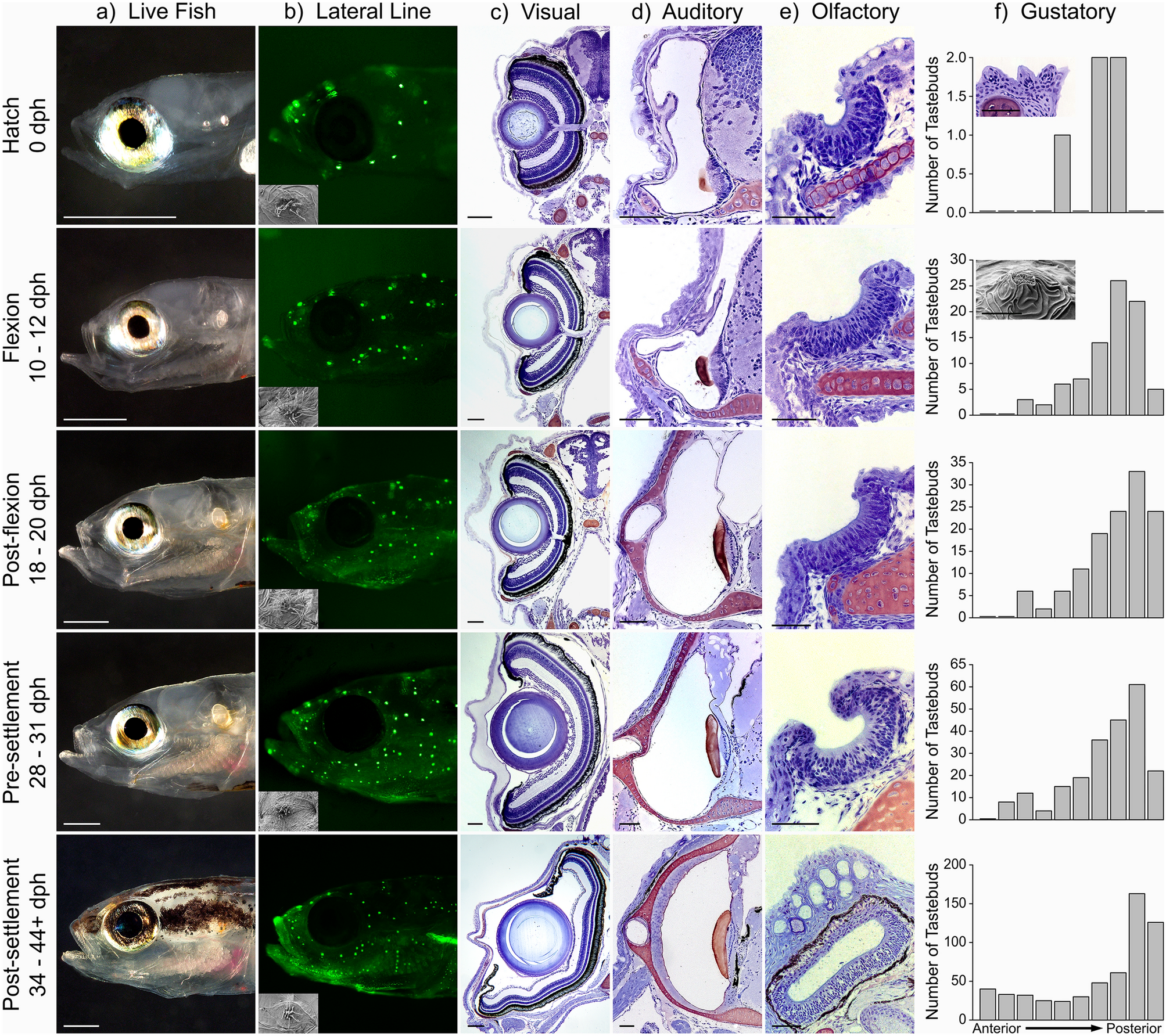
The late stage larvae of coral reef fishes are known to respond to chemosensory, auditory and/or visual cues during navigation and/or settlement. It is known that fish larvae can swim directionally at rates faster than prevailing currents, but the way in which larvae of any one species orients (from hatching to transformation and settlement) has not been investigated. However, the anatomy and function of individual sensory systems has only been examined in a limited number of coral reef fish species. We carried out the first integrated study of the ontogeny of multiple senses and larval orientation behavior, and the first such analysis of the sensory biology of a member of the family Gobiidae, the largest family of reef fishes and one that is a critical component of coral reef fish communities.
 Sensory anatomy of Elacatinus lori settler (9 mm SL). a) Olfactory organ is blind sac with olfactory epithelium (oe), and one of two nares (n) visible. Oral valves (v) in buccal cavity (bc) bounded ventrally by mandible (md). b) Flat olfactory epithelium (oe) with superficial neuromasts (sn) and cupular remnant (blue); c) Eyes with retina (r), lens (l), iris (ir), cornea (c); medial supraorbital LL canal grooves (so). d) Inner ear, lateral to the hindbrain (hb), showing semicircular canal (sc), otolithic organ (saccule, sa), and innervation by auditory nerve (VIII); distance between asterisks indicates otolith size (dissolved in prep.). e) SEM of mandible (ventral view) with neuromasts (arrows). Inset = neuromast with hair cells and orientation (arrow). f) Example of DASPEI-stained neuromasts (dorsal side of head, E. lobeli adult). Scale bars: a, c, d = 100 µm; e = 100 µm (inset = 2 µm), b = 50 µm, f = 1 mm.
Sensory anatomy of Elacatinus lori settler (9 mm SL). a) Olfactory organ is blind sac with olfactory epithelium (oe), and one of two nares (n) visible. Oral valves (v) in buccal cavity (bc) bounded ventrally by mandible (md). b) Flat olfactory epithelium (oe) with superficial neuromasts (sn) and cupular remnant (blue); c) Eyes with retina (r), lens (l), iris (ir), cornea (c); medial supraorbital LL canal grooves (so). d) Inner ear, lateral to the hindbrain (hb), showing semicircular canal (sc), otolithic organ (saccule, sa), and innervation by auditory nerve (VIII); distance between asterisks indicates otolith size (dissolved in prep.). e) SEM of mandible (ventral view) with neuromasts (arrows). Inset = neuromast with hair cells and orientation (arrow). f) Example of DASPEI-stained neuromasts (dorsal side of head, E. lobeli adult). Scale bars: a, c, d = 100 µm; e = 100 µm (inset = 2 µm), b = 50 µm, f = 1 mm.
Sensory Ontogeny in the goby, Elacatinus lori – In Majoris et al., 2021, Scientific Reports
Morphological Methods Used: Histological analysis, vital fluorescent staining, scanning electron microscopy (SEM), µCT imaging, and genomic methods were used to study the development of the olfactory, gustatory, auditory, lateral line, and visual systems.
Data Resources:
- Data archive for entire project (Belize field data, morphological data) at BCO-DMO: https://www.bco-dmo.org/project/651265.
- Histological material (slides; serial sections, stained with cresol violet, 5 µm) and SEM images of ontogenetic series of the following species – available for study in the Webb Lab by prior arrangement: Elacatinus lori , E. colini, Amblyglyphidodon leucogaster, Amphiprion polymnus, Cheilodipterus quinquelineatus.
- SEM images (all but E. lori are AMNH specimens on loan to JFW) available for study in the Webb Lab by prior arrangement: Elacatinus randalli, E. horsti, E. punticulatus; Tigrigobius dilepis, T. inornatus, T. pallens, and T. zebrella, T. gemmatus, T. multifasciatus. Some material has been returned to AMNH.
- µCT data for Elacatinus lori is available for study in the Webb Lab.
Publications:
- Hu Y, Majoris JE, Buston PM, Webb JF. 2018. Potential roles of smell and taste in the orientation behavior of coral-reef fish larvae: Insights from morphology. Journal of Fish Biology – Special Issue on Sensory Ecology of Fishes. 10.1111/jfb.13793.
- Nickles K*, Hu Y, Majoris JE, Buston, PM and Webb JF. 2020. Organization and ontogeny of a complex lateral line system in a goby (Elacatinus lori), with a consideration of function and ecology. Copeia 108(4), 863-885. https://doi.org/10.1643/CG-19-341. Awarded 2020 Best Paper in Ichthyology in Copeia.
- Majoris, J.E., Foretich, M.A., Hu, Y., Nickles, KR,, Di Persia CL, Chaput R, Webb JF, Paris CB, Buston, PM. 2021. An integrative investigation of sensory organ development and orientation behavior throughout the larval phase of a coral reef fish. Sci Rep 11, 12377. https://www.nature.com/articles/s41598-021-91640-2 (Open Access)
- Hu Y, Majoris JE, Buston PM, Webb, JF. 2022. Ear development in select coral reef fishes: Clues for the role of hearing in larval orientation behavior? Ichthyology and Herpetology. 110: 759-775. AWARDED 2022 Best Paper in Ichthyology (Young Investigator) in Ichthyology and Herpetology.
- Foretich MA, Majoris JE, Chaput R, Di Persia CL, Schlatter E, Webb JF, Buston PM, Paris CB. 201_. Larval fish orientation behavior in environmental context. Submitted to Marine Ecology Progress Series. Submitted Nov. 2019.
Conference Presentations:
- Webb, JW. Sensory ontogeny in fishes: It’s all in the timing. Ecology and Evolutionary Ethology of Fishes, Tallahassee, FLA. June 2016.
- Hu Y. and Webb, JF. Ontogeny of the olfactory and gustatory systems in Elacatinus spp. (Gobiidae): Potential for chemosensory-guided navigation in pelagic larvae? Joint Meeting of Ichthyologists and Herpetologists, New Orleans, LA. July 2016.
- Hu, Y, Majoris, JE, Buston, PM, and Webb, JF. 2017. Development of the nose and internal taste buds in two species of neon gobies (Elacatinus spp): Potential to facilitate navigation of pelagic larvae. SICB, New Orleans, LA. Integrative and Comparative Biology. In Press.
- Nickles K, Hu Y, Majoris JE, Buston PM and Webb JF. 2017. Ontogeny of the Lateral Line System in a Caribbean Reef Goby, Elacatinus lori. ASIH/JMIH annual meeting – Austin TX July 2017.
- Webb JF, Hu Y, Nickles KR, Majoris JE, Buston PM. 2018. How do Coral Reef Fish Larvae Find A Home?: Insights From Sensory Organ Ontogeny. 42ndLarval Fish Conference, Victoria BC, Canada, June 2018
- Majoris, JE, Foretich, MA, Hu Y, Chaput R, Schlatter E, Di Persia CL, Webb JF, Paris CB, Buston PM. 2018. Larval orientation behavior begins shortly after hatching in a coral reef fish. 42ndLarval Fish Conference, Victoria BC, Canada, June 2018 (poster)
- Hu Y, Majoris JE, Buston PM, and Webb JF. 2018. Ontogeny of the ear in larval and juvenile coral reef fishes (Gobiidae, Pomacentridae, Apogonidae). Joint Meeting of Ichthyologists and Herpetologists. Rochester NY, July 2018.
- Nickles KR and Webb JF. 2019. Does habitat predict lateral line morphology among species of neon gobies (Genera Elacatinus and Tigrigobius)? SICB, Tampa, FL.
- Molnar, E and Webb JF. 2019. Elaborations of the lateral line system in tetras (Family Characidae: Order Characiformes). SICB, Tampa FL, Jan. 2019.
- Webb JF, Nickles KR, Jones AE. 2019. The Lateral Line System of Larval Teleosts: New Perspectives on Structural and Functional Diversity. 43rdLarval Fish Conference, Mallorca, Spain.
- Webb, JF,Molnar EJ, Nickles KR, Jones AE, McHenry MJ. 2019. Distinguishing Pattern from Chaos: Superficial Neuromast Receptor Organs of the Lateral Line System in Fishes. International Congress of Vertebrate Morphology, Prague, Czech Republic,
- Webb, JF, Molnar EJ, Nickles KR, Jones AE, Conway KW, McHenry MJ. 2020. How to Distinguish Pattern from Chaos: Superficial Neuromasts of the Mechanosensory Lateral Line System in Fishes. SICB, Austin, TX.
- Majoris JE, Foretich MA, Hu, Y, Nickles KR, Di Persia CL, Chaput R, Schlatter E, Webb JF, Paris CB, Buston PM. 2020. Neon Goby Larvae Have Sufficiently Developed Sensory Systems and Swimming Abilities to Orient Directionally Beginning Shortly After Hatching. SICB, Austin, TX.
- Majoris, JE, Foretich, MA, Hu Y, Nickles KR*, Di Persia CL, Chaput R, Schlatter E, Webb JF, Paris CB, Buston PM. 2020. Neon goby larvae have sufficiently developed sensory systems and swimming abilities to orient directionally beginning shortly after hatching. ICRS 2020 – 14th International Coral Reef Symposium, Bremen, Germany.
Post-Docs and Graduate Students Trained:
- Dr. Yinan Hu – Post-Doc (PhD in Craig Albertson lab, U. Mass Amherst) – 2015- 2017 – Sensory ontogeny in Elacatinus spp. (olfaction, gustation, audition, lateral line)
- Katie Nickles – MS student (2017-2019; BS in Marine Biology URI) – Development of the lateral line and visual systems in Elacatinus spp. (see Nickles, et al., 2020 in Copeia)
Undergraduate Researchers:
- Oliver Bender (Marine Biology major, URI) – Summer 2015 – Development of olfactory and gustatory systems in Elacatinus spp. [now MS student at Univ. New Hampshire, Fall 2016]
- Katie Nickles (Marine Biology major, URI) – 2016-2017 – Development of mechanosensory lateral line system in Elacatinus. [then did MS in Webb Lab on this project]
- Elizabeth Molnar (Marine Biology major, URI; CELS Coastal Fellow) – 2018-2019. Fluorescent imaging of the lateral line system in fishes with neuromast proliferations for comparison with gobies.
- Alec Mauk (Marine Biology major, URI) – Summer 2018. Image analysis of goby neuromasts.
